The sodium-ion battery, which was developed at the University of Washington in St. Louis, Missouri, lacks one important element, making it not only cheaper than traditional lithium-ion batteries but also has a significantly smaller size.
“The best anode is no anode,” says Associate Professor Peng Bai, whose lab made the discovery, which is featured in an article in the May issue of Advanced Science.
The very concept of replacing scarce lithium with cheap sodium and even the idea of eliminating the anode were known before, but so far no one has been able to demonstrate the stable operation of an anode-less battery for a more or less practical period of time. The reason leading to the growth of dendrites in such batteries was believed to be the high reactivity of alkali metals, in this case, sodium.
In the new design, only a thin layer of copper foil is used as a current collector on the anode side, i.e. the battery does not have an active anode material. Instead of accumulating in the anode, the ions in the anodeless battery are converted to metal. First, they are deposited on copper foil, and when it comes time to return to the cathode, they dissolve in the electrolyte.
“There are no dendrites in our discovery,” comments Bingyuan Ma, the first author of the article. – The deposited layer is smooth, with a metallic sheen. Such a growth regime has never been observed for an alkali metal of this type. ”
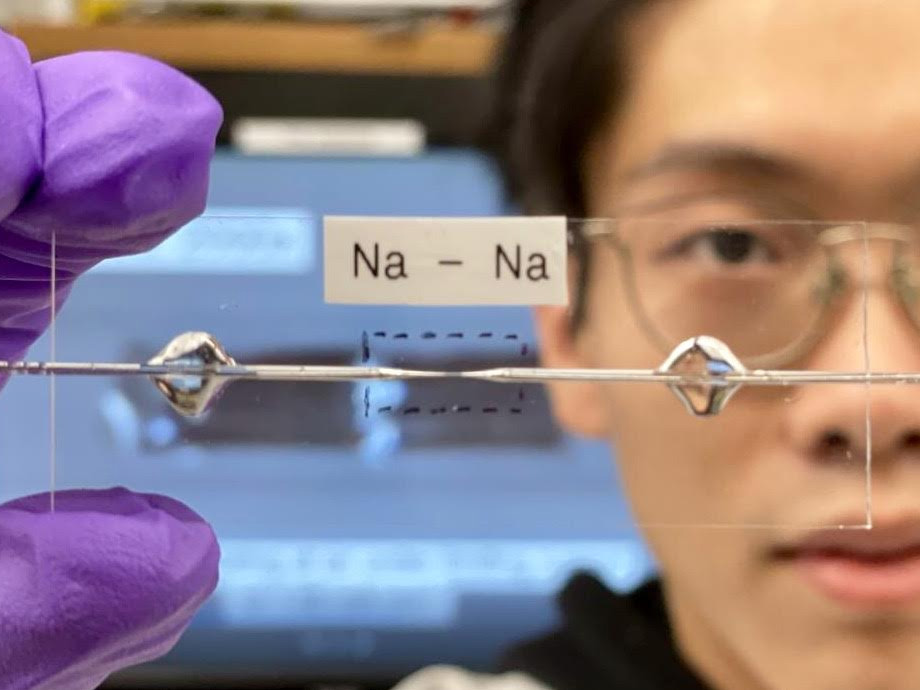
A transparent capillary cell developed by scientists helped to control the state of the battery during its operation. It made it possible to visually observe the emergence of morphological inhomogeneities that serve as embryos for dendrites. It also made it possible to clarify exactly how much it is necessary to reduce the amount of water in the electrolyte (to reduce the reactivity of sodium).
“The water content must be below 10 ppm,” Bai said. With this understanding, he was able to build not just a capillary cell, but a working battery, which in its characteristics is not inferior to a standard lithium-ion one, but takes up much less space due to the lack of an anode.